No products in the cart.
Brio X series has been approved by KFDA
Brio X series has been approved by KFDA
Finally, Bionet’s all-new multi-parameter patient monitoring system, Brio X series, has been approved by KFDA.
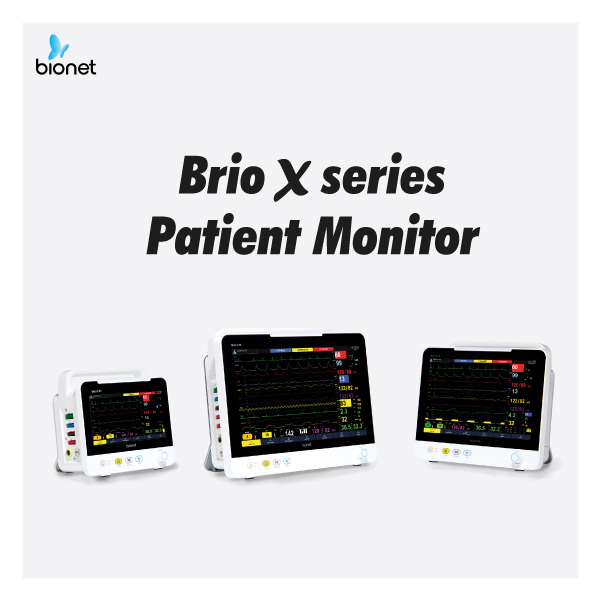
Brio X series has groundbreaking performance, modern design, and friendly usability that all healthcare professionals deserve.Brio X series consists of 3 products: X30, X50, and X70.
All Brio X patient monitors offer 12 Channel ECG, Bionet patented ST Map; and a detachable Paramount with Multi-gas, NMT, EEG, and ICG (Non-invasive cardiac output). The wide spectrum of Brio X functions are suitable for various scenarios: from the ER to intensive care units.Brio X series is currently undergoing the approval process in the United States and Europe.
*The specification published above is based on the Brio X70, our highest tier model within the Brio X series. Brio X70 may include exclusive options that other models may not have.
For detailed specifications regarding each model, please refer to our website.